Isolation, Biochemical Characterization and Anti-adhesion Property of Mucin from the Blue Blubber Jellyfish (Catostylus mosaicus) 
2. Brien Holden Vision Institute, Level 4 North Wing, Rupert Myers Building, Gate 14 Barker Street, University of New South Wales, Kensington, NSW 2052, Australia
3. The School of Optometry and Vision Science, University of New South Wales, Kensington, NSW 2052, Australia
 Author
Author  Correspondence author
Correspondence author
Bioscience Methods, 2011, Vol. 2, No. 4 doi: 10.5376/bm.2011.02.0004
Received: 18 May, 2011 Accepted: 23 May, 2011 Published: 03 Jun., 2011
Pearson et al., 2011, Isolation, biochemical characterization and anti-adhesion property of mucin from the blue blubber jellyfish (Catostylus mosaicus), Bioscience Methods, doi:10.5376/bm.2011.02.0004
Mucins are large glycoproteins that have been identified as the main component of various biological gels and lubricants. Their compositions contribute to the viscoelastic properties of mucus secretions. Gel-forming mucins have now been identified in a variety of organisms from marine molluscs to humans and are thought to cover and protect epithelial cells from attachment and entry of pathogens. We employed a new approach using a combination of tryptic digestion and the fractionation by hydrophobic interaction chromatography to enable the isolation and purification of mucins from the Blue Blubber jellyfish, Catostylus mosaicus. The purified proteins were stained with Alcian blue indicating extensive glycosylation. Amino acid composition analysis of the purified protein found that it was enriched for Ala, Glu, Thr, Pro and Val residues (together constituting ~93 mole % of the protein), which is typical for mucins. Consistent with this, monosaccharide composition analysis revealed extensive O-linked oligosaccharides with N-acetylgalactosamine (GalNAc), galactose (Gal) and N-acetylglucosamine (GlcNAc) as major monosaccharide constituents. The purified C. mosaicus mucins inhibited the attachment of an ocular Pseudomonas aeruginosa (PA) isolate (Paer6294) to human corneal epithelial (HCE) cells grown in vitro.
Jellyfish are found throughout the oceans and seas of the world, ranging from the waters of the Arctic and Antarctic through to the equator. At certain times of the year they swarm in their millions causing problems for the fishing, power generation, shipping and tourism industries. In Eastern and Northern seaboards of Australia the most common jellyfish is the edible Rhizostome, Catostylus mosaicus (commonly referred to as the Blue Blubber jellyfish).
Mucins belong to a family of glycoproteins that contains a large amount of O-linked oligosaccharides. These oligosaccharides are responsible for the viscoelastic properties of mucous secretions and for providing protection of the exposed epithelial surfaces from dehydration, microbial invasion and physical injuries (Vanklinken et al., 1995; Watanabe, 2002). Secreted mucins present in the ocular tear-film of humans have been reported to help maintain fluid viscosity and facilitate lubrication of the eye by retarding fluid evaporation and anchoring the aqueous tear-film to the underlying cornea and conjunctival surfaces (Argüeso and Gipson, 2001; Davidson and Kuonen, 2004; Gipson et al., 2004). In addition, mucins in tear fluid also protect against pathogens by acting as decoy receptors for the binding of pathogens to corneal epithelial surfaces (Mantelli and Argueso, 2008). Human tear fluid which contains a mixture of secreted and shed membrane-associated mucins, was found to be protective against Pseudomonas aeruginosa (Pa)’s colonisation in both cultured corneal epithelial cells (Fleiszig et al., 2003) and in an animal model where corneas were challenged with cytotoxic Pa (Kwong et al., 2007). Epidemiologic evidence suggested that conjunctiva mucin secretion may be impaired in various disorders affecting the ocular surface including dry eye syndrome, Sjogren’s syndrome as well as other types of ocular surface trauma. For example, patients with Sjogren’s syndrome showed a significant decrease in mucin MUC5AC mRNA in a conjunctival epithelial sample and associated reduction in MUC5AC protein in tear samples (Argüeso et al., 2002).
Mucins have recently been extracted from jellyfish. Masuda et al (Masuda et al., 2007) reported isolation and characterization of a novel jellyfish mucin named qniumucin or Q-mucin, with structural similarity to the human MUC5AC mucin. Q-mucin contains unique tandem repeat regions composed of 8 amino acids with a consensus sequence of Val-Val-Glu-Thr- Thr-Ala-Ala-Pro and is heavily glycosylated through N-acetylgalactosamine (GalNAc) O-linkage to the two Thr residues in the tandem repeat. Mucins in general may have potential for wider commercial applications due to their moisture retention, high viscosity and lubrication abilities. For example, they are considered to be potent protective coating substances for use in the field of biomaterials (Chen et al., 2004; Sandberg et al., 2009) due to their resistance to proteolytic degradation and ability to aggregate and form lubricating gels. They are also generally bio- compatible and non-toxic. However, the extensive post-translational modifications of mucins make their synthesis through recombinant protein expression systems very difficult. Moreover, current utilisation of mucins is limited because of the difficulty in extracting and isolating sufficient quantities from most animals. These extractions also typically employ toxic substances such as guanidium chloride (Berry et al., 2003), acetone (Adikwu, 2005) or a large amount of ethanol (Masuda et al., 2007). Suitable extraction procedures for marine mucins such as those from jellyfish, molluscs and squids, which do not involve the use of toxic substances and can be scaled up to factory production level may pave the way for utilising these mucins in the pharmaceutical, food and cosmetics industries. Here we report the extraction, composition analyses and microbial anti-adhesion activity of C. mosaicus mucins.
1 Results
1.1 Isolation and purification of C. mosaicus mucins
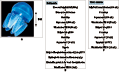
Mucins were extracted from C. mosaicus bell tissue (i.e. the umbrella-shaped body) of healthy jellyfish (Figure 1A). Figure 1B summarizes the steps used in the purification of bell mucin. Following tissue homogenization and sonication, proteins in the supernatant were concentrated by ultra-filtration followed by dialysis against 20 mmol/L Tris-HCl (pH 8.0). During this process, approximately 60% of the non-mucin proteins and a blue pigment precipitated and were subsequently removed by centrifugation (Figure 2, lane B). Extensive glycosylation and the lack of lysine and arginine (specificity determinants for trypsin cleavage) in the repeat domain of previously characterized Q-mucin (Masuda et al., 2007) suggested that C. mosaicus mucin could be trypsin-resistant whereas most other non-mucin-like proteins would be digested by trypsin. Therefore, the supernatant (Figure 1B, supernatant 2) was subjected to tryptic digestion followed by hydrophobic interaction chromatography (HIC). When the break-through fractions from the HIC column were analysed by sodium dodecyl sulfate-polyacrylamide gel electro- phoresis (SDS-PAGE), a large trypsin- resistant (120~300 kD) diffuse band was observed which was strongly stained by Alcian blue (Figure 2, lane C) and poorly with silver or Coomassie blue (data not shown). These staining properties are typical of mucins (Sando et al., 2009). The diffuse nature of the SDS-PAGE band may be due to anomalous electrophoretic behavior caused by substantial glycosylation, which is also characteristic of mucins.
|
|
|
|
We also attempted to purify mucin proteins from the mucus-like exudate released from the jellyfish when suspended in air (Figure 1C). This exudate was characterized by high viscosity and high lubrication abilities (data not shown). Initial dialysis of the concentrated exudate against Tris-HCl (pH 8.0), 150 mmol/L sodium chloride produced a pale light blue precipitate constituting of approximately 30%~ 40% of the total protein in the sample, which was removed by centrifugation. The supernatant was subjected to tryptic digestion followed by ultrafiltration (Figure 1C). The presence of protein was checked by SDS-PAGE. Based on the SDS-PAGE and the Alcian blue staining profiles of the trypsin digested exudate (Figure 2, lane D), the presence of a mucin-like protein could not be confirmed despite the extract’s consistency. No attempt was made to further characterize this material. It is possible that non- proteinacious material could have been responsible for the mucin-like physical properties of the exudate.
The protein concentrations in the bell mucin and exudate mucin extracts were initially determined by using the BCA protein assay with bovine serum albumin as the standard (Pierce Biotechnology IL., USA). This yielded figures of 2.5 mg/jellyfish and 23 mg/jellyfish, respectively. However, this assay may have underestimated the true protein concentration of purified mucins due to their low Trp, Tyr and Cys amino acid contents (Chen et al., 2008). To better determine the yield of mucin, the bell mucin and the exudate mucin were extensively dialyzed against distilled water and a standard volume of each was freeze-dried and their weights measured. These estimates indicated that the yield of purified bell mucin was 72 mg/jellyfish or 0.008% of the bell tissue wet weight. The yield of exudate mucin was 391 mg/ jellyfish or 0.09% of the total jellyfish wet weight.
1.2 Amino acid and monosaccharide compositions
Analysis of the monosaccharide composition of the C. mosaicus bell mucin revealed very high carbohy- drate content (>50% of the dry weight) with N-acety- lgalactosamine (GalNAc), galactose (Gal) and N-ace- tylglucosamine (GlcNAc) as major constituents, and minor amounts of mannose and sialic acid (Table 1). The prevalence of GalNAc, in particular, is consistent with extensive O-linked glycosylation. The excess of GalNAc compared with other monosaccharides indicates that there is substantial O-linked glyco- sylation with only this monosaccharide. The presence of sialic acid is consistent with the alcian blue staining of the mucin and its poor affinity for hydrophobic interaction chromatography. The presence of smaller amounts of N-acetylglucosamine (GlcNAc) and mannose may indicate the existence of either more complex O-glycans or a small quantity of N-linked oligosaccharides. The amino acid composition of the bell mucin was heavily biased with five amino acid residues. Indeed, the sum of Thr, Ala, Val, Pro and Glu residues accounted for 93% of all amino acids (Table 1). An abundance of Thr, Ala, Pro and Glu residues is typical of most mucins (Chen et al., 2008). The approximate equimolar quantities of Thr, Ala, Val and to a lesser extent Pro suggests repetitive tetrapeptide or pentapeptide (if Glu is also included) core structures. This biased composition is also consistent with the tandem octapeptide amino acid composition present in Q-mucin (Masuda et al., 2007). The minor amounts of Lys and Arg reflected the resistance of this mucin to trypsin digestion.
|
|
1.3 Inhibition of bacterial adhesion
To test for bacterial adhesion inhibition activity of the jellyfish mucins, we carried out experiments using the human corneal epithelial (HCE) cell line grown in cell culture and an ocular isolate of Pseudomonas aeruginosa (Paer6264-GFP). Table 2 shows the effects of bell mucin, exudate mucin and bovine MUC1 on the adhesion of Paer6294-GFP to HCE cells following preincubation of the mucins with the bacteria. Bell mucin was the strongest inhibitor of bacterial binding to HCE cells compared to exudate mucin and bovine MUC1. The latter mucin was prepared from bovine milk and has been previously shown to interfere with the binding of bacteria to various animal cells grown in culture (Parker et al., 2010). The number of adhering bacteria was reduced from 10.5×105 cells in the control to 1.4×105 cells when 100 μg/mL of bell mucin was used in the assay, i.e. 86% inhibition. The result also suggested that bell mucin has more anti-bacterial adhesion activity than bovine MUC1. The exudate mucin showed both the rheological properties of mucus (data not shown) and considerable inhibition (26%~55%) of bacterial binding to HCE cells (Table 2).
|
|
Sugars attached to the mucins are the likely candidates binding bacteria and preventing their attachment to HCE cells. Therefore we tested whether the major monosaccharides found in C. mosaicus bell mucin could modulate bacterial binding to HCE cells. When GalNAc, Gal and Glc were used at varying concentrations in the bacterial adhesion assay, both GalNAc and Gal, were able to inhibit bacterial adhesion to HCE cells (Table 3). Binding of bacteria to HCE cells was not affected by Glc.
|
|
2 Discussion
In this study we isolated bell mucin from the blue blubber jellyfish, C. mosaicus, using a combination of trypsin resistance to digestion and hydrophobic interaction chromatography. C. mosaicus bell mucin has high molecular mass (120~300 kD) with oligosaccharides contributing more than 50% of the molecular mass of the glycoprotein (Table 1 and Figure 2). Amino acid and monosaccharide compositional analyses are consistent with a protein composed of tandem repeats containing Val, Thr- GalNAc+ (+ is indicative of a possible elongation of the carbohydrate chain), Ala, Pro and Glu residues with an approximate ratio of 2:2:2:1:1. The results are similar to the amino acid and major monosaccharide constituents of Q-mucin (Masuda et al., 2007). The presence of minor amounts of mannose and sialic acid in C. mosaicus bell mucin (Table 1) indicated the existence of more complex O-linked glycans and/or N-linked glycans. In contrast, the oligosaccharides of Q-mucin contained no sialic acid (Uzawa et al., 2009). Urai et al (Urai et al., 2009) has further characterised the glycan components of Q-mucin and found a unique monosaccharide, 2-amino-ethyl phosphonate (2AEP)- (6)-GalNAc, using a combination of NMR and ESI-MS/MS. This unique O-glycan was not detected by us. However, the ester bond of 2AEP-(6) -GalNAc could not be cleaved by hydrazidation or the hydrolysis method that we used in our mono- saccharide compositional analysis. The elution property (e.g. retention time on HPLC) of this new 2AEP-(6)-GalNAc is very similar to that of GlcNAc.
Therefore it is possible that some or all of the bell mucin GlcNAc may represent this new structure. C. mosaicus bell mucin stained strongly with Alcian blue which is sensitive to acidic sulfonic and carboxy groups. The presence of sialic acid is consistent with this staining pattern. Further research is needed to cha- racterise the full spectrum of glycans on C. mosaicus bell mucin.
Jellyfish mucins have high amino acid compositional and sequence similarity to the human MUC5AC mucin (Masuda et al., 2007), although they appear to contain no (or minimal) Ser residues in any repeat structure. MUC5AC comprises the majority of mucins in the conjunctiva tissue and secretion and in human tear fluid (Argüeso et al., 2002; McKenzie et al., 2000; Jumblatt et al., 1999). MUC5AC are present at much higher levels in human conjunctiva epithelia than those for the secreted mucin MUC2 (McKenzie et al., 2000), and it may be implicated in common eye disorders. MUC5AC mRNA expression in eyes of patients with atopic keratoconjunctivitis (Dogru et al., 2008) and in tears of patients with Sjogren syndrome (Argüeso et al., 2002) was reduced compared with those of healthy individuals. In addition, MUC5AC mRNA production is up-regulated in response to P. aeruginosa and its exoproducts (Li et al., 1998). The current hypothesis regarding mucin function and tear film structure is that the secreted mucins, such as MUC5AC, are part of the “first-line defence” barrier that prevents pathogens from traversing the mucosal barrier (Davidson and Kuonen, 2004; Mantelli and Argüeso, 2008). Our study has demonstrated that jellyfish mucins possess potential binding sites for P. aeruginosa and prevent its binding to epithelial cells grown in cell culture. There has been increasing interest directed toward the protective properties of mucins as a barrier against bacterial attachment to epithelial cells (Alemka et al., 2010; Bergstrom et al., 2010) and the mechanisms by which bacteria can utilise these mucin glycoproteins to facilitate adhesion and colonisation (Vieira et al., 2010; Linden et al., 2009). Our results show that GalNAc and Gal inhibit adhesion of Paer6294-GFP to cultured corneal epithelial cells and suggest that the interaction between bell mucin and P. aeruginosa is glycan- mediated. This observation is consistent with previous studies reporting that the sugars GalNAc and Gal, typically associated with mucins, bind to P. aeruginosa pilus adhesions (Sheth et al., 1994; Ramphal and Arora, 2001). However, different P. aeruginosa strains are known to exhibit different binding specificities to mucins (Aristoteli and Willcox, 2001) and some sugar structures on mucins may be more favourable for bacterial adhesion (Abbeele et al., 2009; Laparra and Sanz, 2009). Our results support this notion. Bovine MUC1 (21% GalNAc, 35% sialic acid) (Sando et al., 2009) showed very strong binding activity to enteric bacteria such as Escherichia coli and Samonella typhimurium (Parker et al., 2010) but bound less effectively to P. aeruginosa than C. mosaicus bell mucin (54% GalNAc, 5% sialic acid) (Table 2). Understanding the interaction between specific bacteria and mucins is required in order to optimise their protective binding properties.
Jellyfish are an untapped resource of easily harvested mucins and other bioactives such as collagen, phospholipids and sphingophosphonolipids. Mucins are found in abundance and in almost every organ of jellyfish (Masuda et al., 2007) and we have demonstrated here that a considerable amount of mucin could be harvested from jellyfish (0.01% wet weight for bell mucin). Interestingly, the crude exu- date which requires very simple and low cost extra- ction can yield a very high amount (0.1% wet weight) of material (presumably complex oligosaccharides) that have high bacterial adhesion inhibitory activity.
Our understanding of the biological functions of jellyfish mucin is rudimentary. Jellyfish are known to secret mucus to help clean their surface and to discourage attacks by predators (Hanaoka et al., 2001). In addition and like their mammalian counterparts, mucins secreted by jellyfish together with their interacting proteins may function to mediate various cellular activities to enhance physical protection, enhance tissue integrity and enhance non-immune host defence (Senapati et al., 2010). These activities allow the jellyfish to survive in their aqueous habitat.
In conclusion, a new and simple protocol was devised to isolate mucins from jellyfish. Both the highly purified material from bell and the crude exudate mucins showed anti-bacterial adherence effects when tested using an ocular isolate of P. aeruginosa. The anti-Pseudomonas adhesion property of C. mosaicus bell mucin probably occurs via interactions involving bacterial proteins and the carbohydrate moieties of the mucin. Jellyfish may be rich sources of novel mucins exhibiting many different biological and mechano- physical activities.
3 Materials and Methods
3.1 Jellyfish
Mature healthy Catostylus mosaicus specimens were collected from the clear waters of Moreton Bay, Queensland during the height of their spawning cycle (October/November). The jellyfish were drained of excess sea water and transported to the laboratory where the tentacles and the bell were separated prior to freezing at -20℃. External mucus-like fluid (exudate) released from the jellyfish during transport to the laboratory, was also collected and frozen at -20℃.
3.2 Mucin extraction from jellyfish bell
Two frozen bells (total weight 1 900 g) were thawed, homogenised for 2 min using an Ultra-Turrax homo- geniser (Janke and Kunkel laboratories, Germany) and sonicated (Misonix Incorporated NY, USA) in 4×450 mL aliquots using a 7.0 mm probe for 30 s at maximum power. To prevent proteolysis and microbial growth, benzamidine hydrochloride (Sigma-Aldrich, Australia), EDTA and sodium azide were added to the homogenate to a final concentration of 1 mmol/L, 1 mmol/L and 0.04%, respectively and the sample centrifuged at 70 000×g for 40 min at 4℃. The super- natant was concentrated by ultra filtration YM 30 (Millipore Corp, MA, USA) to 100 mL prior to exten- sive dialysis against 20 mmol/L TrisHCl (pH 8.0). The sample was again centrifuged as above to remove the resultant heavy blue precipitate and the total protein content of the clear supernatant determined by using the BCA assay (Pierce Biotechnology, IL, USA). Trypsin (Porcine, Sigma-Aldrich) was added at an enzyme to substrate (Figure 1, supernatant 2) ratio of 1:20 to the concentrated supernatant and the solution digested for 3 h at 37℃. Digestion was halted by the addition of benzamidine hydrochloride, EDTA (and sodium azide) as indicated above and the solution further concentrated to a final volume of 36 mL. This strategy relies on the relative resistance of mucins to tryptic digestion.
3.3 Hydrophobic interaction chromatography (HIC)
15 mL (packed column volume) of high substitution Phenyl Sepharose (GE Healthcare USA) was used to fractionate the soluble extract. The trypsin digested concentrate was dialysed against 50 mmol/L sodium phosphate (pH 7.0), 0.8 mol/L ammonium sulphate and applied to the column in 6×6 mL aliquots. The break-through fractions were collected, pooled and bound proteins recovered from the resin with a 10 mL pulse of 50 mmol/L sodium phosphate (pH 7.0). SDS-PAGE was performed on all fractions collected from the HIC column to confirm the presence of trypsin-resistant proteins. Pooled break-through fractions from the HIC purification were dialysed against PBS and used for compositional analyses and bacterial adhesion assays. The protein in the break- through fractions stained strongly with Alcian blue after SDS-PAGE and hence it was called bell mucin.
3.4 Protein extraction from external mucus-like fluid (exudate mucin)
Nine hundred ml of external mucus-like fluid, released from the jellyfish during collection, was thawed and concentrated to 46 mL by using an ultra filtration YM 10 (Millipore Corp). The concentrate was dialysed against 50 mmol/L Tris-HCl (pH 8.0), 150 mmol/L sodium chloride and any precipitant removed by centrifugation (70 000×g, 40 min, 4℃). The total protein content of the supernatant was determined using the BCA assay (Pierce Biotechnology), and the sample was then digested with trypsin as described above. Digestion was halted by addition of benzamidine hydrochloride, EDTA (and sodium azide) at final concentrations as indicated above and then the sample was concentrated to 23 mL, dialysed against phosphate buffered saline (PBS) and frozen at -20℃.
3.5 Amino acid and monosaccharide compositional analyses
For amino acid and monosaccharide compositional analyses, the HIC break-through fractions rich in mucin-like protein were pooled, dialysed against 50 mmol/L TrisHCl (pH 8.0), 150 mmol/L sodium chloride and further purified by gel permeation chromatography (GPC) on a TSK-Gel G3000SW 7.5 mm×30 cm column (Tosoh Corporation Japan). Fractions eluting in the void volume were collected, pooled, concentrated and dialysed against distilled water and amino acid analysis performed by the Australian Proteome Analysis Facility Ltd., (Macquarie University, Sydney, Australia) using stan- dard protocols (Sando et al., 2009). Cys and Trp contents were not determined. Monosaccharide com- positional analysis was determined by the Glyco- technology Core Resource Facility, (University of California, San Diego, USA) using chromatographic techniques (Sando et al., 2009).
3.6 Bacterial culture
A corneal isolate of P. aeruginosa (strain Paer6294- GFP) was used in the study. This strain was originally isolated from an infectious corneal ulcer (Aristoteli and Willcox, 2001) and then engineered to harbour a plasmid expressing green fluorescent protein (GFP) (Christensen et al., 2007). This allowed the detection of bacteria by fluorescence. The strain was taken from -86℃storage and subcultured once on chocolate blood agar plates (Micro Diagnostics, Brisbane, QLD, Australia) containing 200 µg/mL carbenicillin at 35℃ for 18 h. The day before use, bacterial colonies were resuspended in 0.01 mol/L PBS (pH 7.4). They were then centrifuged at 3 000×g for 10 min, washed once with PBS, pelleted and resuspended in Minimum Essential Medium Eagle (MEM, Life Technologies, Grand island, NY, USA) supplemented with 0.6% (w/v) bovine serum albumin (BSA, Sigma-Aldrich) and 0.035% (w/v) NaHCO3.
3.7 Cell culture
Human corneal epithelial (HCE) cells were cultured in modified SHEM (supplemented hormone epithelial medium) which is MEM supplemented with 5%~10% (v/v) foetal bovine serum (FBS), 1.5 mmol/L L-glutamine (Invitrogen), 50 µg/mL gentamicin (Invitrogen), 1 µg/mL of cholera toxin (Sigma-Aldrich, St Louise, MO, USA), 0.1 µg/mL of murine epidermal growth factor (Sigma-Aldrich) and 5 µg/mL insulin from bovine pancreas (Sigma-Aldrich). Monolayers of HCE cells were grown at 37℃in a humidified atmosphere of 5% CO2 in T25 or T75 tissue culture flasks (Becton- Dickenson Labware, Oxnard, CA, USA).
3.8 Bacterial adhesion assay
100 µL of 5×106 colony forming units (CFU)/mL Paer6294-GFP was mixed with 100 µL of serially diluted mucins (50~200 µg/mL for bell mucin or 62.5~500 µg/mL for exudate mucin). The bacteria were allowed to bind to mucin at 20℃ for 2 h with agitation. The mixture (100 µL) was then added to HCE cells (1×105/well) grown in black 96-well plates (Greiner Bio-One GmbH, Germany), and allowed to incubate at 37℃ for 1 h. BSA at the highest concentration of mucin (100 µg /mL) was used as the negative control. All assays were performed in duplicate and the experiment repeated twice. Unbound bacteria were removed by washing the wells with PBS (×2) and the GFP fluorescence intensity of each well was measured (emission 535 nm, excitation 485 nm) using a Tecan SpectroFluor Plus. The numbers of adherent Paer6294-GFP in the wells were interpolated from a standard curve, which was run with each experiment. Briefly, 100 µL serial dilutions of Paer6294-GFP (100×105, 50×105, 25×105, 12.5×105, 6.25×105, 3.12×105, 1.56×105 and 0.78×105 CFU/mL) were transferred in duplicates into a black 96-well plate containing 1×105/well of HCE cells. The bacteria were allowed to bind to the cells at 37℃ for 1 h after which the cells were washed twice with PBS and the fluorescence data were measured. A standard curve was created by plotting the mean fluorescence data for each concentration against bacterial concentration.
3.9 Monosasccharide inhibition
The monosaccharides used in this assay represented those major monosaccharides present on bell mucin as determined by the compositional analysis described above. They were tested in a bacterial anti-adhesion assay. D (+) galactose (Gal), N-acetylgalactosamine (GalNAc), and D (+) glucose (Glc) (Sigma-Aldrich) were prepared as 50 mmol/L, 25 mmol/L and 12.5 mmol/L solutions in PBS. 200 μL of each mono- saccharide solution was incubated with 200 μL of Paer6294-GFP (1×108 CFU/mL) in MEM in the wells of a 24-well plate and then incubated for 1 h at 20℃with gentle shaking. Following this incubation, 100 μL of the Paer6294-GFP and monosaccharide mixture was applied to HCE cells (seeded at 2.5× 105/well) and incubated for 1 h (37℃, 5% CO2) in a 96-well plate. Following 3 washes with MEM, fluorescence intensities in wells were measured and the number of adherent bacteria was calculated from a standard curve, constructed as described above.
Authors’ contributions
RP carried out the mucin extraction and purification, participated in compositional analyses, and contributed to the writing, RT participated in the design of the study and edited the manuscript, RA performed the cytotoxicity test. BX and ZZ performed bacterial adherence assays and analysed the data. MW coordinated the project and helped to edit the manuscript. KK conceived the study, and participated in the experimental design and coordination and wrote the manuscript. All authors read, and approved the final manuscript.
Acknowledgements
We thank Gene Wijffels and Michelle Colgrave for the review of the manuscript.
References
Abbeele P.V.D., Grootaert C., Possemiers S., Verstraete W., Verbeken K., and Wiele T.V.D., 2009, In vitro model to study the modulation of the mucin-adhered bacterial community, Appl. Microbiol. Biotechnol., 83(2): 349-359
doi:10.1007/s00253-009-1947-2 Mid:19308405
Adikwu M.U., 2005, Evaluation of snail mucin motifs as rectal absorption enhancer for insulin in non-diabetic rat models, Biol. Pharm. Bull., 28(9): 1801-1804 doi:10.1248/bpb.28.1801
Alemka A., Whelan S., Gough R., Clyne M., Gallagher M.E., Carrington S.D., and Bourke B., 2010, Purified chicken intestinal mucin attenuates Campylobacter jejuni pathogenicity in vitro, J. Med. Microbiol., 59(8): 898-903 doi:10.1099/jmm.0.019315-0 PMid:20466838
Argüeso P., Balaram M., Spurr-Michaud S., Keutmann H.T., Dana M.R., and Gipson I.K., 2002, Decreased levels of the goblet cell mucin MUC5AC in tears of patients with Sjögren syndrome, Invest. Ophthalmol. Vis. Sci., 43(4): 1004-1011 PMid:11923240
Argüeso P., and Gipson I.K., 2001, Epithelial mucins of the ocular surface: Structure, biosynthesis and function, Exp. Eye. Res., 73(3): 281-289 doi:10.1006/exer.2001.1045 PMid:11520103
Aristoteli L.P., and Willcox M.D.P., 2001, Adhesion of Pseudomonas aeruginosa ocular isolates to mucin, Clin. Experiment. Ophthalmol., 29(3): 143-146 doi:10.1046/j.1442-9071.2001.00395.x PMid:11446454
Bergstrom K.S.B., Kissoon-Singh V., Gibson D.L., Ma C.X., Montero M., Sham H.P., Ryz N., Huang T.N., Velcich A., Finlay B.B., Chadee K., and Vallance B.A., 2010, Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa, Plos Pathog., 6(5): e1000902 doi:10.1371/journal.ppat.1000902 PMid:20485566 PMCid:2869315
Berry M., Harris A., and Corfield A.P., 2003, Patterns of mucin adherence to contact lenses, Invest. Ophthalmol. Vis. Sci., 44(2): 567-572 doi:10.1167/iovs.02-0720
Chen X., Lee G.S., Zettl A., and Bertozzi C.R., 2004, Biomimetic engineering of carbon nanotubes by using cell surface mucin mimics, Angew. Chem. Int. Ed. Engl., 43(45): 6111-6116 doi:10.1002/anie.200460620 PMid:15549753
Chen Y.Z., Tang Y.R., Sheng Z.Y., and Zhang Z.D., 2008, Prediction of mucin-type O-glycosylation sites in mammalian proteins using the composition of k-spaced amino acid pairs, BMC Bioinformatics, 9: 101 doi:10.1186/1471-2105-9-101 PMid:18282281 PMCid:2335299
Christensen L.D., Moser C., Jensen P. Ø., Rasmussen T.B., Christophersen L., Kjelleberg S., Kumar N., HØiby N., Givskov M., and Bjarnsholt T., 2007, Impact of Pseudomonas aeruginosa quorum sensing on biofilm persistence in an in vivo intraperitoneal foreign-body infection model, Microbiology, 153(Pt 7): 2312-2320 doi:10.1099/mic.0.2007/006122-0 PMid:17600075
Davidson H.J., and Kuonen V.J., 2004, The tear film and ocular mucins, Vet. Ophthalmol., 7(2):71-77 doi:10.1111/j.1463-5224.2004.00325.x PMid:14982585
Dogru M., Matsumoto Y., Okada N., Igarashi A., Fukagawa K., Shimazaki J., Tsubota K., and Fujishima H., 2008, Alterations of the ocular surface epithelial MUC16 and goblet cell MUC5AC in patients with atopic keratoconjunctivitis, Allergy, 63(10): 1324-1334 doi:10.1111/j.1398-9995.2008.01781.x PMid:18782111
Fleiszig S.M.J., Kwong M.S.F., and Evans D.J., 2003, Modification of Pseudomonas aeruginosa interactions with corneal epithelial cells by human tear fluid, Infect. Immun., 71(7): 3866-3874 doi:10.1128/IAI.71.7.3866-3874.2003
PMid:12819071 PMCid:162005
Gipson I.K., Hori Y., and Argüeso P., 2004, Character of ocular surface mucins and their alteration in dry eye disease, Ocul. Surf., 2(2): 131-148(18)
Hanaoka K., Ohno H., Wada N., Ueno S., Goessler W., Kuehnelt D., Schlagenhaufen C., Kaise T., and Irgolic K.J., 2001, Occurrence of organo-arsenicals in jellyfishes and their mucus, Chemosphere, 44(4): 743-749 doi:10.1016/S0045-6535(00)00291-5
Jumblatt M.M., McKenzie R.W., Jumblatt J.E., 1999, MUC5AC mucin is a component of the human precorneal tear film, Invest. Ophthalmol. Vis. Sci., 40(1): 43-49 PMid:9888425
Kwong M.S.F., Evans D.J., Ni M.J., Cowell B.A., and Fleiszig S.M.J., 2007, Human tear fluid protects against Pseudomonas aeruginosa keratitis in a murine experimental model, Infect. Immun., 75(5): 2325-2332 doi:10.1128/IAI.01404-06 PMid:17325054 PMCid:1865794
Laparra J.M., and Sanz Y., 2009, Comparison of in vitro models to study bacterial adhesion to the intestinal epithelium, Lett. Appl. Microbiol., 49(6): 695-701 doi:10.1111/j.1472-765X.2009.02729.x PMid:19843211
Li D.Z., Gallup M., Fan N., Szymkowski D.E., and Basbaum C.B., 1998, Cloning of the amino-terminal and 5'-flanking region of the human MUC5AC mucin gene and transcriptional up-regulation by bacterial exoproducts, J. Biol. Chem., 273(12): 6812-6820 doi:10.1074/jbc.273.12.6812 PMid:9506983
Lindén S.K., Sheng Y.H., Every A.L., Miles K.M., Skoog E.C., Florin T.H.J., Sutton P., and McGuckin M.A., 2009, MUC1 limits Helicobacter pylori infection both by steric hindrance and by acting as a releasable decoy, Plos Pathog., 5(10): e1000617 doi:10.1371/journal.ppat.1000617 PMid:19816567 PMCid:2752161
Mantelli F., and Argüeso P., 2008, Functions of ocular surface mucins in health and disease, Curr. Opin. Allergy. Clin. Immunol., 8(5):477-483 doi:10.1097/ACI.0b013e32830e6b04 PMid:18769205 PMCid:2666617
Masuda A., Baba T., Dohmae N., Yamamura M., Wada H., and Ushida K., 2007, Mucin (qniumucin), a glycoprotein from jellyfish, and determination of its main chain structure, J. Nat. Prod., 70(7): 1089-1092 doi:10.1021/np060341b
PMid:17567171
McKenzie R.W., Jumblatt J.E., and Jumblatt M.M., 2000, Quantification of MUC2 and MUC5AC transcripts in human conjunctiva, Invest. Ophthalmol. Vis. Sci., 41(3): 703-708 PMid:10711684
Parker P., Sando L., Pearson R., Kongsuwan K., Tellam R.L., and Smith S., 2010, Bovine Muc1 inhibits binding of enteric bacteria to Caco-2 cells, Glycoconj. J., 27(1): 89-97 doi:10.1007/s10719-009-9269-2 PMid:19936918
Ramphal R., and Arora S.K., 2001, Recognition of mucin components by Pseudomonas aeruginosa, Glycoconj. J., 18(9): 709-713 doi:10.1023/A:1020823406840 PMid:12386456
Sandberg T., Karlsson Ott M., Carlsson J., Feiler A., and Caldwell K.D., 2009, Potential use of mucins as biomaterial coatings.II. Mucin coatings affect the conformation and neutrophil-activating properties of adsorbed host proteins—toward a mucosal mimic, J. Biomed. Mater. Res., 91A(3): 773-785 doi:10.1002/jbm.a.32315 PMid:19051307
Sando L., Pearson R., Gray C., Parker P., Hawken R., Thomson P.C., Meadows J.R.S., Kongsuwan K., Smith S., and Tellam R.L., 2009, Bovine Muc1 is a highly polymorphic gene encoding an extensively glycosylated mucin that binds bacteria, J. Dairy Sci., 92(10): 5276-5291 doi:10.3168/jds.2009-2216 PMid:19762846
Senapati S., Das S., and Batra S.K., 2010, Mucin-interacting proteins: From function to therapeutics, Trends Biochem. Sci., 35(4): 236-245 doi:10.1016/j.tibs.2009.10.003 PMid:19913432 PMCid:3030310
Sheth H.B., Lee K.K., Wong W.Y., Srivastava G., Hindsgaul O., Hodges R.S., Paranchych W., and Irvin R.T., 1994, The pili of Pseudomonas aeruginosa strains PAK and PAO bind specifically to the carbohydrate sequence ßGalNAc (1-4) ßGal found in glycosphingolipids asialo-GM1 and asialo-GM2, Mol. Microbiol., 11(4): 715-723 doi:10.1111/j.1365-2958.1994.tb00349.x PMid:7910939
Urai M., Nakamura T., Uzawa J., Baba T., Taniguchi K., Seki H., and Ushida K., 2009, Structural analysis of O-glycans of mucin from jellyfish (Aurelia aurita) containing 2-aminoethylphosphonate, Carbohyd. Res., 344(16): 2182-2187
doi:10.1016/j.carres.2009.08.001 PMid:19732869
Uzawa J., Urai M., Baba T., Seki H., Taniguchi K., and Ushida K., 2009, NMR study on a novel mucin from jellyfish in natural abundance, qniumucin from Aurelia aurita, J. Nat. Prod., 72(5): 818-823 doi:10.1021/np800601j PMid:19371080
van klinken B.J., Dekker J., Buller H.A., and Einerhand A.W., 1995, Mucin gene structure and expression: Protection vs. adhesion, Am. J. Physiol-Gastr. L., 269(5): G613-G627
Vieira M.A,M., Gomes T.A.T., Ferreira A.J,P., Knöbl T., Servin A.L., and Liévin-Le Moal V. 2010, Two atypical enteropathogenic Escherichia coli strains induce the production of secreted and membrane-bound mucins to benefit their own growth at the apical surface of human mucin-secreting intestinal HT29-MTX cells, Infect, Immun,m 78(3): 927-938
Watanabe H., 2002, Significance of mucin on the ocular surface, Cornea, 21 (2 Suppl 1): S17-S22 doi:10.1097/00003226-200203001-00005 PMid:11995804
. PDF(308KB)
. FPDF(win)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Roger Pearson
. Ross Tellam
. Banglao Xu
. Zhenjun Zhao
. Mark Willcox
. Kritaya Kongsuwan
Related articles
. Mucin
. Glycosylation
. Protein purification
. Bacterial anti-adhesion
. Jellyfish
Tools
. Email to a friend
. Post a comment
.png)





